Find
Search for over 200,000 study notes and past assignments!
Swap
Download study resources by swapping your own or buying Exchange Credits.
Study
Study from your library anywhere, anytime.
24 Pages • Essays / Projects • Year Uploaded: 2021
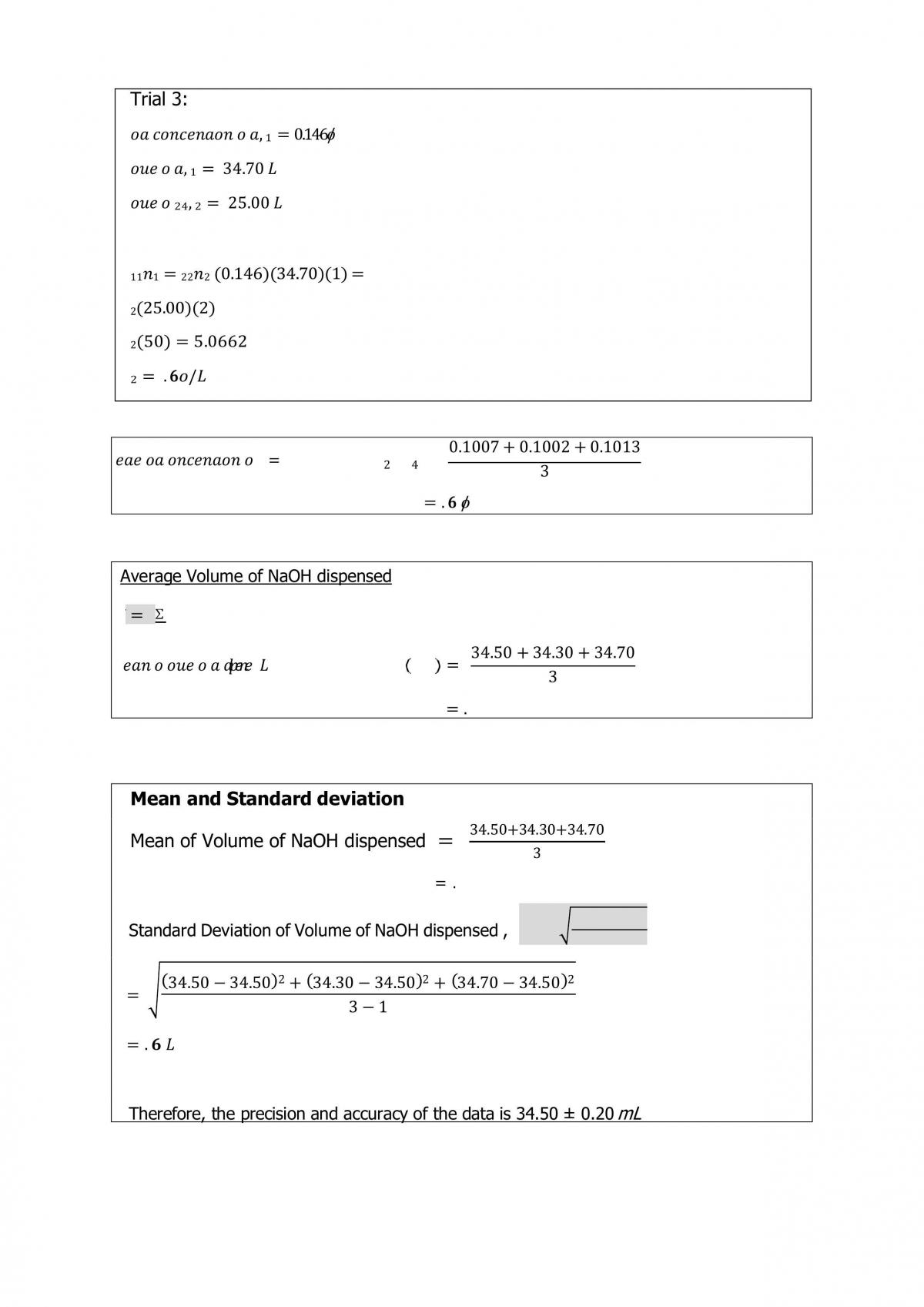
Primary standards are those chemical reagents having high percent purity, about 99%, stable towards the air, have high molecular mass, readily soluble in the solvent as well as readily available. A standard solution is a solution in which its concentration is accurately known. The standard solution that reacts with the substance is being analyzed. Primary standard and standard solution are typically used in titration to determine an unknown concentration. During the standardization of NaOH solution, potassium hydrogen phthalate, KHC8H4O4 acts as the primary acid standard which determines the molar concentration of a sodium hydroxide. After standardizing the NaOH solution, it is called a secondary standard solution. Titration is a common laboratory method of quantitative chemical analysis that is used to determine the unknown concentration of an identified analyte (Medwick & Kirschner, 2010). In this experiment, a buret is used to dispense a the standardized NaOH solution (titrant) into a flask containing the acid solution (analyte). An indicator is used to signal the stoichiometric point during the titration. The phenolphthalein indicator is used whereby it is colorless when it is added into the acid solution and it turns into pink as soon as the solution is basic, as soon as the pink solution is formed, the titration
This document is 10 Exchange Credits
More about this document:
|
|
This document has been hand checkedEvery document on Thinkswap has been carefully hand checked to make sure it's correctly described and categorised. No more browsing through piles of irrelevant study resources. |
|
|
This is an Essay / ProjectEssays / Projects are typically greater than 5 pages in length and are assessments that have been previously submitted by a student for academic grading. |
|
|
What are Exchange Credits?Exchange Credits represent the worth of each document on Thinkswap. In exchange for uploading documents you will receive Exchange Credits. These credits can then be used to download other documents for free. |
|
|
Satisfaction GuaranteeWe want you to be satisfied with your learning, that’s why all documents on Thinkswap are covered by our Satisfaction Guarantee. If a document is not of an acceptable quality or the document was incorrectly described or categorised, we will provide a full refund of Exchange Credits so that you can get another document. For more information please read Thinkswap's Satisfaction Guarantee. |

Studying with Academic Integrity
Studying from past student work is an amazing way to learn and research, however you must always act with academic integrity.
This document is the prior work of another student. Thinkswap has partnered with Turnitin to ensure students cannot copy directly from our resources. Understand how to responsibly use this work by visiting ‘Using Thinkswap resources correctly’.
Browse UMS Subjects